US EPA uses TD–GC–MS for vital research into PFAS management
23 February 2021
PFAS – per- and polyfluoroalkyl substances – have been making headlines in recent years. Documentaries and films such as the 2019 legal thriller Dark Waters, which is based on real events, have also raised their profile. As a result, many of us have heard of PFAS but what exactly are they and why are they of concern?
What are PFAS?
PFAS are a large family of chemicals with over 6000 registered species.1 They’re typically defined as aliphatic compounds with one or more carbon atoms where all the hydrogens have been replaced by fluorine atoms.2 Given this broad definition, we can understand why so many compounds are classed as PFAS.
As of 2021, two PFAS compounds – PFOS and PFOA (Figure 1) – are already restricted for use and production by the Stockholm convention with a third – PFHxS – expected to be given the same restrictions. PFOA is described as “a substance of very high concern with a persistent, bioaccumulative and toxic structure for the environment and living organisms.” This gives an insight into the wider concerns regarding PFAS as a group of chemicals.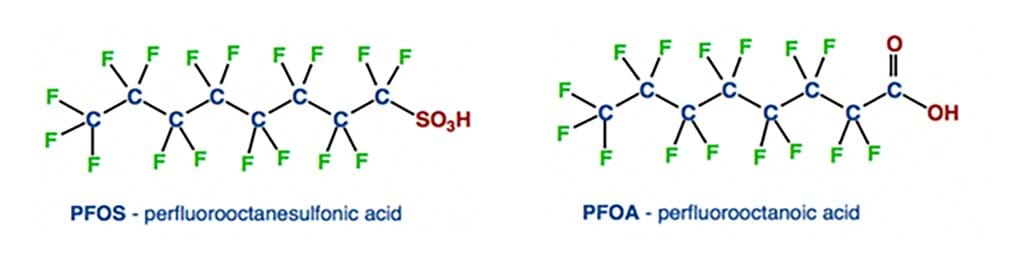
Figure 1: Chemical structures of PFOS (left) and PFOA (right).
However, PFAS are useful compounds that are found in many items in our homes and workplaces, such as non-stick pans and waterproof clothing. They are also used, for example, in semiconductors and firefighting foams. So, as well as limiting the release of these compounds into the air, water and soil as they are being manufactured, how can regulators manage the safe disposal of products containing PFAS?
This was the focus of a US EPA paper published in February 2021.3 Titled “Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide”, the paper focuses on the neutral PFAS species fluorotelomer alcohols and uses two analytical techniques – chemical ionisation mass spectrometry (CIMS) and thermal desorption (TD) coupled to gas chromatography (GC)–mass spectrometry (MS) – to monitor the compounds’ breakdown during incineration. The aim was to optimise the process so it can occur at lower temperatures, reducing the risk of PFAS compounds being released into the air due to incomplete breakdown.
TD–GC–MS overcomes the challenges of using LC–MS
From an analytical perspective, the paper is interesting, not just because it discusses a potential solution to the release of PFAS into the air we breathe, but also that using TD–GC–MS in this application area is not commonplace, and the paper debunks some common myths around sampling gaseous PFAS compounds.
PFAS monitoring in gaseous samples has mostly been carried out on LC–MS rather than GC–MS. However, more volatile PFAS, such as the fluorotelomer alcohols, can be a challenge for LC. Equally, sorbent sampling media, such as PUF and XAD, which have been used for sampling gases, also struggle to capture more volatile species. In this respect, the move to TD–GC–MS makes sense as the technique is already used to monitor organic compounds in air, including ultra-volatile species.
Another advantage of moving away from LC–MS for gaseous samples is that the need to use solvents is avoided, in both the chromatographic system and as part of the sample extraction process. If you’ve listened to PFAS conference or webinar presentations from established laboratories over the past few years, you will have heard about challenges surrounding PFAS contamination of both solvents and sampling media. TD–GC–MS is a solvent-free technique so this wasn’t something that the authors of the US EPA paper had to contend with, although other sources of PFAS contamination is still something analysts have to be mindful of.
A possible source of PFAS contamination in many labs is PTFE, which is used in tubing, for example. PTFE has long been vilified in the LC world because solvents cause it to leach from materials. The EPA researchers wanted to verify that PTFE leaching wouldn’t be an issue in their investigation. They collected blanks from the furnace and sampling train, which included PTFE fittings and transfer lines, to verify that no fluorinated compounds were being introduced. When analysed on the Markes TD100-xr™, none of the blank samples contained fluorinated compounds, confirming that the PTFE fittings and the sorbent media within the tubes were suitable for use in PFAS sampling.
Benefit of the non-selective nature of TD sampling
As with many PFAS monitoring and research projects, the EPA paper is focused on target compounds. However, the researchers point out the non-selective nature of the TD sampling approach as a benefit, not just for looking at target compounds but also to see what else is being produced during the treatment process, including other PFAS. Here, PFAS fragments were indicative of incomplete destruction and could have been missed by more targeted techniques. Additionally, other non-PFAS VOCs could be monitored within the same experiment.
If you’re interested in PFAS research and the analytical techniques used to monitor them, I would encourage you to read the paper using the link below. It’s an open access paper and well worth a read. In the meantime, if you are interested in discussing the use of TD–GC–MS for analysis of volatile PFAS from air or materials, please get in touch.
https://doi.org/10.1016/j.chemosphere.2021.129859
- https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster.
- ITRC, Technical/Regulatory Guidance, Per- and Polyfluoroalkyl substances (PFAS), April 2020, page 3.
- T.P. Riedel, M. Ariel Geer Wallace, E.P. Shields, J.V. Ryan, C.W. Lee and W.P. Linak, Low Temperature Thermal Treatment of Gas-Phase Fluorotelomer Alcohols by Calcium Oxide, Chemosphere, 2021, 129859, https://doi.org/10.1016/j.chemosphere.2021.129859.